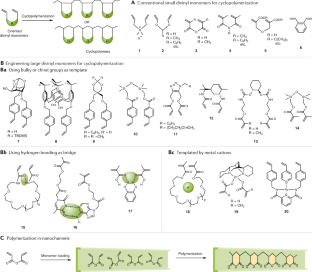
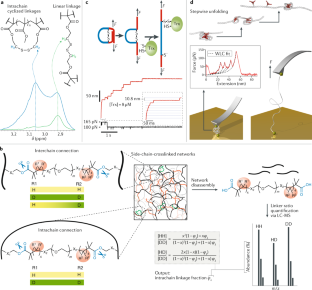
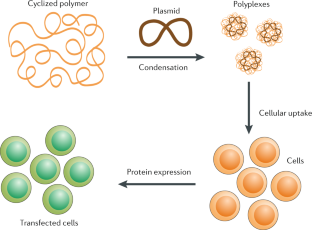
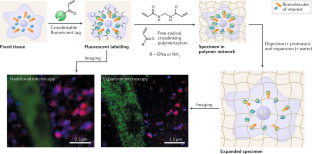
Complex polymer architectures through free-radical polymerization of multivinyl monomers
The construction of complex polymer architectures with well-defined topology, composition and functionality has been extensively explored as the molecular basis for the development of modern polymer materials. The unique reaction kinetics of free-radical polymerization leads to the concurrent formation of crosslinks between polymer chains and rings within an individual chain and, thus, free-radical (co)polymerization of multivinyl monomers provides a facile method to manipulate chain topology and functionality. Regulating the relative contribution of these intermolecular and intramolecular chain-propagation reactions is the key to the construction of architecturally complex polymers. This can be achieved through the design of new monomers or by spatially or kinetically controlling crosslinking reactions. These mechanisms enable the synthesis of various polymer architectures, including linear, cyclized, branched and star polymer chains, as well as crosslinked networks. In this Review, we highlight some of the contemporary experimental strategies to prepare complex polymer architectures using radical polymerization of multivinyl monomers. We also examine the recent development of characterization techniques for sub-chain connections in such complex macromolecules. Finally, we discuss how these crosslinking reactions have been engineered to generate advanced polymer materials for use in a variety of biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
133,45 € per year
only 11,12 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
One-step synthesis of sequence-controlled multiblock polymers with up to 11 segments from monomer mixture
Article Open access 10 January 2022
From controlled radical polymerization of vinyl ether to polymerization-induced self-assembly
Article 26 August 2022

Highly robust supramolecular polymer networks crosslinked by a tiny amount of metallacycles
Article Open access 09 April 2024
References
- PlasticsEurope. Plastics — the Facts 2018https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf (2018).
- Nesvadba, P. in Encyclopedia of Radicals in Chemistry, Biology and Materialshttps://doi.org/10.1002/9781119953678.rad080 (Wiley, 2012).
- Matyjaszewski, K. & Tsarevsky, N. V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem.1, 276–288 (2009). CASPubMedGoogle Scholar
- Matyjaszewski, K. Architecturally complex polymers with controlled heterogeneity. Science333, 1104–1105 (2011). CASPubMedGoogle Scholar
- Georges, M. K., Veregin, R. P. N., Kazmaier, P. M. & Hamer, G. K. Narrow molecular weight resins by a free-radical polymerization process. Macromolecules26, 2987–2988 (1993). CASGoogle Scholar
- Wang, J.-S. & Matyjaszewski, K. Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc.117, 5614–5615 (1995). CASGoogle Scholar
- Chiefari, J. et al. Living free-radical polymerization by reversible addition–fragmentation chain transfer: the RAFT process. Macromolecules31, 5559–5562 (1998). CASGoogle Scholar
- Stals, P. J. M. et al. How far can we push polymer architectures? J. Am. Chem. Soc.135, 11421–11424 (2013). CASPubMedGoogle Scholar
- Newland, B. et al. Single cyclized molecule versus single branched molecule: a simple and efficient 3D “knot” polymer structure for nonviral gene delivery. J. Am. Chem. Soc.134, 4782–4789 (2012). CASPubMedGoogle Scholar
- Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nat. Mater.13, 645–652 (2014). CASPubMedPubMed CentralGoogle Scholar
- Terashima, T., Kamigaito, M., Baek, K.-Y., Ando, T. & Sawamoto, M. Polymer catalysts from polymerization catalysts: direct encapsulation of metal catalyst into star polymer core during metal-catalyzed living radical polymerization. J. Am. Chem. Soc.125, 5288–5289 (2003). CASPubMedGoogle Scholar
- Staudinger, H. & Husemann, E. Über hochpolymere Verbindungen, 116. Mitteil.: Über das begrenzt quellbare Poly-styrol. Ber. Dtsch. Chem. Ges.68, 1618–1634 (1935). Google Scholar
- Flory, P. J. Molecular size distribution in three dimensional polymers. I. Gelation. J. Am. Chem. Soc.63, 3083–3090 (1941). CASGoogle Scholar
- Stockmayer, W. H. Theory of molecular size distribution and gel formation in branched polymers II. General cross linking. J. Chem. Phys.12, 125–131 (1944). CASGoogle Scholar
- Chen, F. F., Tillberg, P. W. & Boyden, E. S. Expansion microscopy. Science347, 543–548 (2015). This work shows the application of free-radical copolymerization of monovinyl/divinyl monomers for biological imaging. CASPubMedPubMed CentralGoogle Scholar
- Distefano, G. et al. Highly ordered alignment of a vinyl polymer by host–guest cross-polymerization. Nat. Chem.5, 335–341 (2013). This work describes the free-radical copolymerization of monovinyl/divinyl monomers in porous coordination polymers, yielding a pseudo-crystalline polymeric network. CASPubMedGoogle Scholar
- Gu, Y., Zhao, J. & Johnson, J. A. A (macro)molecular-level understanding of polymer network topology. Trends Chem.1, 318–334 (2019). Google Scholar
- Seiffert, S. Origin of nanostructural inhomogeneity in polymer-network gels. Polym. Chem.8, 4472–4487 (2017). CASGoogle Scholar
- Matsumoto, A., Miwa, Y., Inoue, S., Enomoto, T. & Aota, H. Discussion of “greatly delayed gelation from theory in free-radical cross-linking multivinyl polymerization accompanied by microgel formation” based on multiallyl polymerization. Macromolecules43, 6834–6842 (2010). CASGoogle Scholar
- Okay, O., Kurz, M., Lutz, K. & Funke, W. Cyclization and reduced pendant vinyl group reactivity during the free-radical crosslinking polymerization of 1,4-divinylbenzene. Macromolecules28, 2728–2737 (1995). CASGoogle Scholar
- Norisuye, T. et al. Small angle neutron scattering studies on structural inhomogeneities in polymer gels: irradiation cross-linked gels vs chemically cross-linked gels. Polymer43, 5289–5297 (2002). CASGoogle Scholar
- Adibnia, V. & Hill, R. J. Universal aspects of hydrogel gelation kinetics, percolation and viscoelasticity from PA-hydrogel rheology. J. Rheol.60, 541–548 (2016). CASGoogle Scholar
- Polanowski, P., Jeszka, J. K., Li, W. & Matyjaszewski, K. Effect of dilution on branching and gelation in living copolymerization of monomer and divinyl cross-linker: modeling using dynamic lattice liquid model (DLL) and Flory–Stockmayer (FS) model. Polymer52, 5092–5101 (2011). CASGoogle Scholar
- Lyu, J. et al. Monte Carlo simulations of atom transfer radical (homo)polymerization of divinyl monomers: applicability of Flory–Stockmayer theory. Macromolecules51, 6673–6681 (2018). CASGoogle Scholar
- Elliott, J. E., Anseth, J. W. & Bowman, C. N. Kinetic modeling of the effect of solvent concentration on primary cyclization during polymerization of multifunctional monomers. Chem. Eng. Sci.56, 3173–3184 (2001). CASGoogle Scholar
- Cerid, H. & Okay, O. Minimization of spatial inhomogeneity in polystyrene gels formed by free-radical mechanism. Eur. Polym. J.40, 579–587 (2004). CASGoogle Scholar
- Elliott, J. E. & Bowman, C. N. Effects of solvent quality during polymerization on network structure of cross-linked methacrylate copolymers. J. Phys. Chem. B106, 2843–2847 (2002). CASGoogle Scholar
- Doura, M., Naka, Y., Aota, H. & Matsumoto, A. Control of intermolecular cross-linking reaction in free-radical cross-linking monovinyl/divinyl copolymerizations by the aid of amphiphilic nature of primary polymer chains and cross-link units with opposite polarities. Macromolecules38, 5955–5963 (2005). CASGoogle Scholar
- Mori, H. & Tsukamoto, M. RAFT polymerization of diacrylate derivatives having different spacers in dilute conditions. Polymer52, 635–645 (2011). CASGoogle Scholar
- Yu, Q., Zhu, Y., Ding, Y. & Zhu, S. Reaction behavior and network development in RAFT radical polymerization of dimethacrylates. Macromol. Chem. Phys.209, 551–556 (2008). CASGoogle Scholar
- Van Camp, W., Gao, H., Du Prez, F. E. & Matyjaszewski, K. Effect of crosslinker multiplicity on the gel point in ATRP. J. Polym. Sci. A Polym. Chem.48, 2016–2023 (2010). Google Scholar
- Elliott, J. E. & Bowman, C. N. Monomer functionality and polymer network formation. Macromolecules34, 4642–4649 (2001). CASGoogle Scholar
- Patras, G., Qiao, G. G. & Solomon, D. H. Novel cross-linked homogeneous polyacrylamide gels with improved separation properties: Investigation of the cross-linker functionality. Electrophoresis22, 4303–4310 (2001). CASPubMedGoogle Scholar
- Denisin, A. K. & Pruitt, B. L. Tuning the range of polyacrylamide gel stiffness for mechanobiology applications. ACS Appl. Mater. Interfaces8, 21893–21902 (2016). CASPubMedGoogle Scholar
- Shi, Y., Graff, R. W., Cao, X., Wang, X. & Gao, H. Chain-growth click polymerization of AB2 monomers for the formation of hyperbranched polymers with low polydispersities in a one-pot process. Angew. Chem. Int. Ed.54, 7562–7562 (2015). Google Scholar
- O’Brien, N., McKee, A., Sherrington, D. C. C., Slark, A. T. T. & Titterton, A. Facile, versatile and cost effective route to branched vinyl polymers. Polymer41, 6027–6031 (2000). This work proposes the ‘Strathclyde route’ for synthesizing branched polymers from radical polymerization of multivinyl monomers with the addition of chain-transfer agents. Google Scholar
- Besenius, P., Slavin, S., Vilela, F. & Sherrington, D. C. Synthesis and characterization of water-soluble densely branched glycopolymers. React. Funct. Polym.68, 1524–1533 (2008). CASGoogle Scholar
- Baudry, R. & Sherrington, D. C. Synthesis of highly branched poly(methyl methacrylate)s using the “Strathclyde methodology” in aqueous emulsion. Macromolecules39, 1455–1460 (2006). CASGoogle Scholar
- Chisholm, M., Hudson, N., Kirtley, N., Vilela, F. & Sherrington, D. C. Application of the “Strathclyde route” to branched vinyl polymers in suspension polymerization: architectural, thermal, and rheological characterization of the derived branched products. Macromolecules42, 7745–7752 (2009). CASGoogle Scholar
- Xiang, L., Song, Y., Qiu, M. & Su, Y. Synthesis of branched poly(butyl acrylate) using the Strathclyde method in continuous-flow microreactors. Ind. Eng. Chem. Res.58, 21312–21322 (2019). CASGoogle Scholar
- Guan, Z. Control of polymer topology through transition-metal catalysis: synthesis of hyperbranched polymers by cobalt-mediated free radical polymerization. J. Am. Chem. Soc.124, 5616–5617 (2002). CASPubMedGoogle Scholar
- McEwan, K. A. & Haddleton, D. M. Combining catalytic chain transfer polymerisation (CCTP) and thio-Michael addition: enabling the synthesis of peripherally functionalised branched polymers. Polym. Chem.2, 1992–1999 (2011). CASGoogle Scholar
- Smeets, N. M. B. Amphiphilic hyperbranched polymers from the copolymerization of a vinyl and divinyl monomer: the potential of catalytic chain transfer polymerization. Eur. Polym. J.49, 2528–2544 (2013). CASGoogle Scholar
- Kurochkin, S. A., Silant’ev, M. A., Perepelitsyna, E. O. & Grachev, V. P. Synthesis of branched polymers via radical copolymerization under oxygen inflow. Eur. Polym. J.57, 202–212 (2014). CASGoogle Scholar
- Hirano, T., Kamiike, R., Hsu, Y., Momose, H. & Ute, K. Multivariate analysis of 13 C NMR spectra of branched copolymers prepared by initiator-fragment incorporation radical copolymerization of ethylene glycol dimethacrylate and tert-butyl methacrylate. Polym. J.48, 793–800 (2016). CASGoogle Scholar
- Liang, S., Li, X., Wang, W.-J. J., Li, B.-G. & Zhu, S. Toward understanding of branching in RAFT copolymerization of methyl methacrylate through a cleavable dimethacrylate. Macromolecules49, 752–759 (2016). CASGoogle Scholar
- Bannister, I., Billingham, N. C., Armes, S. P., Rannard, S. P. & Findlay, P. Development of branching in living radical copolymerization of vinyl and divinyl monomers. Macromolecules39, 7483–7492 (2006). CASGoogle Scholar
- Rosselgong, J., Armes, S. P., Barton, W. & Price, D. Synthesis of highly branched methacrylic copolymers: observation of near-ideal behavior using RAFT polymerization. Macromolecules42, 5919–5924 (2009). CASGoogle Scholar
- Bannister, I., Billingham, N. C. & Armes, S. P. Monte Carlo modelling of living branching copolymerisation of monovinyl and divinyl monomers: comparison of simulated and experimental data for ATRP copolymerisation of methacrylic monomers. Soft Matter5, 3495–3504 (2009). CASGoogle Scholar
- Bouhier, M.-H., Cormack, P. A. G., Graham, S. & Sherrington, D. C. Synthesis of densely branched poly(methyl methacrylate)s via ATR copolymerization of methyl methacrylate and ethylene glycol dimethacrylate. J. Polym. Sci. A Polym. Chem.45, 2375–2386 (2007). CASGoogle Scholar
- Liu, B., Kazlauciunas, A., Guthrie, J. T. & Perrier, S. One-pot hyperbranched polymer synthesis mediated by reversible addition fragmentation chain transfer (RAFT) polymerization. Macromolecules38, 2131–2136 (2005). CASGoogle Scholar
- Gao, H., Min, K. & Matyjaszewski, K. Determination of gel point during atom transfer radical copolymerization with cross-linker. Macromolecules40, 7763–7770 (2007). CASGoogle Scholar
- Yang, H. et al. Synthesis of highly branched polymers by reversible complexation-mediated copolymerization of vinyl and divinyl monomers. Polym. Chem.8, 2137–2144 (2017). CASGoogle Scholar
- Flynn, S., Dwyer, A. B., Chambon, P. & Rannard, S. Expanding the monomer scope of linear and branched vinyl polymerisations via copper-catalysed reversible-deactivation radical polymerisation of hydrophobic methacrylates using anhydrous alcohol solvents. Polym. Chem.10, 5103–5115 (2019). CASGoogle Scholar
- Rosselgong, J., Armes, S. P., Barton, W. R. S. & Price, D. Synthesis of branched methacrylic copolymers: comparison between RAFT and ATRP and effect of varying the monomer concentration. Macromolecules43, 2145–2156 (2010). CASGoogle Scholar
- Gao, Y., Newland, B., Zhou, D., Matyjaszewski, K. & Wang, W. Controlled polymerization of multivinyl monomers: formation of cyclized/knotted single-chain polymer architectures. Angew. Chem. Int. Ed.56, 450–460 (2017). CASGoogle Scholar
- Wang, W. et al. Controlling chain growth: a new strategy to hyperbranched materials. Macromolecules40, 7184–7194 (2007). CASGoogle Scholar
- Zhao, T. et al. Water soluble hyperbranched polymers from controlled radical homopolymerization of PEG diacrylate. RSC Adv.5, 33823–33830 (2015). CASGoogle Scholar
- Zhao, T., Zheng, Y., Poly, J. & Wang, W. Controlled multi-vinyl monomer homopolymerization through vinyl oligomer combination as a universal approach to hyperbranched architectures. Nat. Commun.4, 1873 (2013). This work describes a universal approach to synthesize hyperbranched polymers from kinetically controlled multivinyl monomers. PubMedGoogle Scholar
- Odian, G. in Principles of Polymerization (Wiley, 2004).
- Gao, H., Miasnikova, A. & Matyjaszewski, K. Effect of cross-linker reactivity on experimental gel points during ATRcP of monomer and cross-linker. Macromolecules41, 7843–7849 (2008). CASGoogle Scholar
- Nagelsdiek, R., Mennicken, M., Maier, B., Keul, H. & Höcker, H. Synthesis of polymers containing cross-linkable groups by atom transfer radical polymerization: poly(allyl methacrylate) and copolymers of allyl methacrylate and styrene. Macromolecules37, 8923–8932 (2004). CASGoogle Scholar
- Yhaya, F., Sutinah, A., Gregory, A. M., Liang, M. & Stenzel, M. H. RAFT polymerization of vinyl methacrylate and subsequent conjugation via enzymatic thiol-ene chemistry. J. Polym. Sci. A Polym. Chem.50, 4085–4093 (2012). CASGoogle Scholar
- Akiyama, M., Yoshida, K. & Mori, H. Controlled synthesis of vinyl-functionalized homopolymers and block copolymers by RAFT polymerization of vinyl methacrylate. Polymer55, 813–823 (2014). CASGoogle Scholar
- Ma, J., Cheng, C., Sun, G. & Wooley, K. L. Well-defined polymers bearing pendent alkene functionalities via selective RAFT polymerization. Macromolecules41, 9080–9089 (2008). CASPubMedPubMed CentralGoogle Scholar
- Ma, J., Cheng, C. & Wooley, K. L. Cycloalkenyl-functionalized polymers and block copolymers: syntheses via selective RAFT polymerizations and demonstration of their versatile reactivity. Macromolecules42, 1565–1573 (2009). CASGoogle Scholar
- Qu, Q., Liu, G., Lv, X., Zhang, B. & An, Z. In situ cross-linking of vesicles in polymerization-induced self-assembly. ACS Macro Lett.5, 316–320 (2016). CASGoogle Scholar
- Chen, L. et al. Chemoselective RAFT polymerization of a trivinyl monomer derived from carbon dioxide and 1,3-butadiene: from linear to hyperbranched. Macromolecules50, 9598–9606 (2017). CASGoogle Scholar
- Dong, Z. M., Liu, X. H., Tang, X. L. & Li, Y. S. Synthesis of hyperbranched polymers with pendent norbornene functionalities via raft polymerization of a novel asymmetrical divinyl monomer. Macromolecules42, 4596–4603 (2009). CASGoogle Scholar
- Ren, J. M. et al. Star polymers. Chem. Rev.116, 6743–6836 (2016). CASPubMedGoogle Scholar
- Gao, H. & Matyjaszewski, K. Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linkers: from stars to gels. Prog. Polym. Sci.34, 317–350 (2009). CASGoogle Scholar
- Wang, D. et al. Kinetics and modeling of semi-batch RAFT copolymerization with hyperbranching. Macromolecules45, 28–38 (2012). This work describes the synthesis of branched polymers from semi-batch copolymerization of multivinyl monomers. CASGoogle Scholar
- Wang, D., Wang, W.-J., Li, B.-G. & Zhu, S. Semibatch RAFT polymerization for branched polyacrylamide production: effect of divinyl monomer feeding policies. AIChE J.59, 1322–1333 (2013). CASGoogle Scholar
- Xia, J., Zhang, X. & Matyjaszewski, K. Synthesis of star-shaped polystyrene by atom transfer radical polymerization using an ‘arm first’ approach. Macromolecules32, 4482–4484 (1999). CASGoogle Scholar
- Gao, H., Ohno, S., & Matyjaszewski, K. Low Polydispersity Star Polymers via Cross-Linking Macromonomers by ATRP. J. Am. Chem. Soc.128, 15111–15113 (2006). CASPubMedGoogle Scholar
- Connal, L. A., Vestberg, R., Hawker, C. J. & Qiao, G. G. Synthesis of dendron functionalized core cross-linked star polymers. Macromolecules40, 7855–7863 (2007). CASGoogle Scholar
- Hatton, F. L., Chambon, P., McDonald, T. O., Owen, A. & Rannard, S. P. Hyperbranched polydendrons: a new controlled macromolecular architecture with self-assembly in water and organic solvents. Chem. Sci.5, 1844–1853 (2014). CASGoogle Scholar
- Hern, F. Y., Hill, A., Owen, A. & Rannard, S. P. Co-initiated hyperbranched-polydendron building blocks for the direct nanoprecipitation of dendron-directed patchy particles with heterogeneous surface functionality. Polym. Chem.9, 1767–1771 (2018). CASGoogle Scholar
- Kanaoka, S., Sawamoto, M. & Higashimura, T. Star-shaped polymers by living cationic polymerization. 1. Synthesis of star-shaped polymers of alkyl and vinyl ethers. Macromolecules24, 2309–2313 (1991). CASGoogle Scholar
- Gao, H. & Matyjaszewski, K. Structural control in ATRP synthesis of star polymers using the arm-first method. Macromolecules39, 3154–3160 (2006). CASGoogle Scholar
- Baek, K.-Y. Y., Kamigaito, M. & Sawamoto, M. Star-shaped polymers by metal-catalyzed living radical polymerization. 1. Design of Ru(II)-based systems and divinyl linking agents. Macromolecules34, 215–221 (2001). CASGoogle Scholar
- Baek, K.-Y., Kamigaito, M. & Sawamoto, M. Core-functionalized star polymers by transition metal-catalyzed living radical polymerization. 1. Synthesis and characterization of star polymers with PMMA arms and amide cores. Macromolecules34, 7629–7635 (2001). CASGoogle Scholar
- Li, W. & Matyjaszewski, K. Star polymers via cross-linking amphiphilic macroinitiators by AGET ATRP in aqueous media. J. Am. Chem. Soc.131, 10378–10379 (2009). CASPubMedGoogle Scholar
- Zhang, X., Xia, J. & Matyjaszewski, K. End-functional poly(tert-butyl acrylate) star polymers by controlled radical polymerization. Macromolecules33, 2340–2345 (2000). CASGoogle Scholar
- Pasquale, A. J. & Long, T. E. Synthesis of star-shaped polystyrenes via nitroxide-mediated stable free-radical polymerization. J. Polym. Sci. A Polym. Chem.39, 216–223 (2001). CASGoogle Scholar
- Gao, H. & Matyjaszewski, K. Synthesis of star polymers by a new “core-first” method: sequential polymerization of cross-linker and monomer. Macromolecules41, 1118–1125 (2008). CASGoogle Scholar
- Butler, G. B. & Bunch, R. L. Preparation and polymerization of unsaturated quaternary ammonium compounds. J. Am. Chem. Soc.71, 3120–3122 (1949). CASGoogle Scholar
- Butler, G. B. & Ingley, F. L. Preparation and polymerization of unsaturated quaternary ammonium compounds. II. Halogenated allyl derivatives. J. Am. Chem. Soc.73, 895–896 (1951). CASGoogle Scholar
- Pasini, D. & Takeuchi, D. Cyclopolymerizations: synthetic tools for the precision synthesis of macromolecular architectures. Chem. Rev.118, 8983–9057 (2018). CASPubMedGoogle Scholar
- Saito, Y. & Saito, R. The effect of the distance between neighboring vinyl groups on template polymerization. Polymer52, 3565–3569 (2011). CASGoogle Scholar
- Matsumoto, A. et al. Crystal engineering for topochemical polymerization of muconic esters using halogen–halogen and CH/π interactions as weak intermolecular interactions. J. Am. Chem. Soc.124, 8891–8902 (2002). CASPubMedGoogle Scholar
- Crawshaw, A. & Butler, G. B. The formation of linear polymers from diene monomers by a cyclic polymerization mechanism. II. Polyacrylic anhydride and the derived polyacrylic acid. J. Am. Chem. Soc.80, 5464–5466 (1958). CASGoogle Scholar
- Tsukino, M. & Kunitake, T. Radical cyclopolymerization of divinyl acetals — structure variation with polymerization conditions. Polym. J.17, 943–951 (1985). CASGoogle Scholar
- Costa, L., Chiantore, O. & Guaita, M. Free radical polymerization of unconjugated dienes: 19. Temperature dependence of the cyclopolymerization of o-divinylbenzene. Polymer19, 202–204 (1978). CASGoogle Scholar
- Jones, R. G., Harry Cragg, R. & Swain, A. C. Structure and mechanism in the cyclopolymerization of diallylsilanes. Eur. Polym. J.28, 651–655 (1992). CASGoogle Scholar
- Erkoc, S., Mathias, L. J. & Acar, A. E. Cyclopolymerization of tert-butyl α-(hydroxymethyl) acrylate (TBHMA) ether dimer via atom transfer radical polymerization (ATRP). Macromolecules39, 8936–8942 (2006). CASGoogle Scholar
- Erkoc, S. & Acar, A. E. Controlled/living cyclopolymerization of tert-butyl α-(hydroxymethyl) acrylate ether dimer via reversible addition fragmentation chain transfer polymerization. Macromolecules41, 9019–9024 (2008). CASGoogle Scholar
- Yokota, K., Matsumura, M., Yamaguchi, K. & Takada, Y. Synthesis of polymers with benzo-19-crown-6 units via cyclopolymerization of divinyl ethers. Macromol. Rapid Commun.4, 721–724 (1983). CASGoogle Scholar
- Wulff, G., Schmidt, H., Witt, H. & Zentel, R. Cooperativity and transfer of chirality in liquid-crystalline polymers. Angew. Chem. Int. Ed.33, 188–191 (1994). Google Scholar
- Kakuchi, T. et al. Chirality induction in cyclocopolymerization. 14. Template effect of 1,2-cycloalkanediol in the cyclocopolymerization of bis(4-vinylbenzoate)s with styrene. Macromolecules34, 38–43 (2001). CASGoogle Scholar
- Zhao, X. & Liu, H. Synthesis and characterization of PEG polymer brushes via cyclopolymerization of 1,2,3-triazole tethered diacrylates. Chin. J. Chem. Phys.28, 739–745 (2015). CASGoogle Scholar
- Ochiai, B., Shiomi, T. & Yoshita, H. Cyclopolymerization of a bisacrylate through selective formation of a 19-membered ring. Polym. J.48, 859–862 (2016). CASGoogle Scholar
- Ochiai, B., Ootani, Y. & Endo, T. Controlled cyclopolymerization through quantitative 19-membered ring formation. J. Am. Chem. Soc.130, 10832–10833 (2008). CASPubMedGoogle Scholar
- Kim, T.-H. et al. Cyclopolymerisation of an oriented 4,6-bis(4-vinylbenzyl)-myo-inositol orthoformate. Chem. Commun.24, 2419–2420 (2000). Google Scholar
- Kim, T.-H., Holmes, A. B. & Giles, M. Ring closing metathesis of a 4,6-diallyl-myo-inositol orthoformate as a model for an inositol cyclopolymer. Chem. Commun.24, 2419–2420 (2000). Google Scholar
- Li, J., Du, M., Zhao, Z. & Liu, H. Cyclopolymerization of disiloxane-tethered divinyl monomers to synthesize chirality-responsive helical polymers. Macromolecules49, 445–454 (2016). CASGoogle Scholar
- Saito, Y. & Saito, R. Synthesis of syndiotactic poly(methacrylic acid) by free-radical polymerization of the pseudo-divinyl monomer formed with methacrylic acid and catechol. J. Appl. Polym. Sci.128, 3528–3533 (2013). CASGoogle Scholar
- Kang, Y., Lu, A., Ellington, A., Jewett, M. C. & O’Reilly, R. K. Effect of complementary nucleobase interactions on the copolymer composition of RAFT copolymerizations. ACS Macro Lett.2, 581–586 (2013). CASGoogle Scholar
- Kimura, Y., Miyabara, Y., Terashima, T. & Sawamoto, M. Polyacrylamide pseudo crown ethers via hydrogen bond-assisted cyclopolymerization. J. Polym. Sci. A Polym. Chem.54, 3294–3302 (2016). CASGoogle Scholar
- Mathur, A. M. & Scranton, A. B. Synthesis and ion-binding properties of polymeric pseudocrown ethers II: template ion induced cyclization of oligomeric ethylene glycol diacrylates. Sep. Sci. Technol.32, 285–301 (1997). CASGoogle Scholar
- Terashima, T., Kawabe, M., Miyabara, Y., Yoda, H. & Sawamoto, M. Polymeric pseudo-crown ether for cation recognition via cation template-assisted cyclopolymerization. Nat. Commun.4, 2321 (2013). This work presents the cyclopolymerization of divinyl monomers in the presence of cation template. PubMedGoogle Scholar
- Jana, S. & Sherrington, D. C. Transfer of chirality from (−)-sparteine zinc(II) (meth)acrylate complexes to the main chains of their (meth)acrylate polymer derivatives. Angew. Chem. Int. Ed.44, 4804–4808 (2005). CASGoogle Scholar
- Hibi, Y., Ouchi, M. & Sawamoto, M. Sequence-regulated radical polymerization with a metal-templated monomer: repetitive ABA sequence by double cyclopolymerization. Angew. Chem. Int. Ed.50, 7434–7437 (2011). This work describes the synthesis of sequence-controlled polymers via cyclopolymerization of multivinyl monomers. CASGoogle Scholar
- Mochizuki, S., Kitao, T. & Uemura, T. Controlled polymerizations using metal–organic frameworks. Chem. Commun.54, 11843–11856 (2018). CASGoogle Scholar
- Uemura, T., Hiramatsu, D., Kubota, Y., Takata, M. & Kitagawa, S. Topotactic linear radical polymerization of divinylbenzenes in porous coordination polymers. Angew. Chem. Int. Ed.46, 4987–4990 (2007). CASGoogle Scholar
- Uemura, T. et al. Controlled cyclopolymerization of difunctional vinyl monomers in coordination nanochannels. Macromolecules47, 7321–7326 (2014). CASGoogle Scholar
- Gao, Y. et al. Intramolecular cyclization dominating homopolymerization of multivinyl monomers toward single-chain cyclized/knotted polymeric nanoparticles. Macromolecules48, 6882–6889 (2015). CASGoogle Scholar
- Elliott, J. E. & Bowman, C. N. Kinetics of primary cyclization reactions in cross-linked polymers: an analytical and numerical approach to heterogeneity in network formation. Macromolecules32, 8621–8628 (1999). CASGoogle Scholar
- Taylor, W. R. & Lin, K. Protein knots: a tangled problem. Nature421, 25 (2003). CASPubMedGoogle Scholar
- Zheng, Y. et al. 3D single cyclized polymer chain structure from controlled polymerization of multi-vinyl monomers: beyond Flory–Stockmayer theory. J. Am. Chem. Soc.133, 13130–13137 (2011). This work describes the synthesis of single-cyclized polymers from intramolecular cyclization-dominated polymerization of multivinyl monomers. CASPubMedGoogle Scholar
- Li, Y. & Armes, S. P. Synthesis and chemical degradation of branched vinyl polymers prepared via ATRP: use of a cleavable disulfide-based branching agent. Macromolecules38, 8155–8162 (2005). CASGoogle Scholar
- Rosselgong, J. & Armes, S. P. Quantification of intramolecular cyclization in branched copolymers by 1 H NMR spectroscopy. Macromolecules45, 2731–2737 (2012). CASGoogle Scholar
- Zhou, H. et al. Counting primary loops in polymer gels. Proc. Natl Acad. Sci. USA109, 19119–19124 (2012). CASPubMedGoogle Scholar
- Wang, J. et al. Counting secondary loops is required for accurate prediction of end-linked polymer network elasticity. ACS Macro Lett.7, 244–249 (2018). CASGoogle Scholar
- Zhong, M., Wang, R., Kawamoto, K., Olsen, B. D. & Johnson, J. A. Quantifying the impact of molecular defects on polymer network elasticity. Science353, 1264–1268 (2016). This work proposes a technique, called symmetric isotopic labelling disassembly spectrometry (SILDaS), to quantify the intrachain links (loops) in polymer networks. CASPubMedGoogle Scholar
- Wang, J. et al. Counting loops in sidechain-crosslinked polymers from elastic solids to single-chain nanoparticles. Chem. Sci.10, 5332–5337 (2019). CASPubMedPubMed CentralGoogle Scholar
- Wang, D. & Russell, T. P. Advances in atomic force microscopy for probing polymer structure and properties. Macromolecules51, 3–24 (2018). CASGoogle Scholar
- Pavliček, N. & Gross, L. Generation, manipulation and characterization of molecules by atomic force microscopy. Nat. Rev. Chem.1, 0005 (2017). Google Scholar
- Krieg, M. et al. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys.1, 41–57 (2019). Google Scholar
- Burdyńska, J. et al. Synthesis and arm dissociation in molecular stars with a spoked wheel core and bottlebrush arms. J. Am. Chem. Soc.136, 12762–12770 (2014). PubMedGoogle Scholar
- Lafferentz, L. et al. Conductance of a single conjugated polymer as a continuous function of its length. Science323, 1193–1197 (2009). CASPubMedGoogle Scholar
- Chung, J., Kushner, A. M., Weisman, A. C. & Guan, Z. Direct correlation of single-molecule properties with bulk mechanical performance for the biomimetic design of polymers. Nat. Mater.13, 1055–1062 (2014). CASPubMedGoogle Scholar
- Beedle, A. E. M. et al. Forcing the reversibility of a mechanochemical reaction. Nat. Commun.9, 3155 (2018). PubMedPubMed CentralGoogle Scholar
- Wiita, A. P. et al. Probing the chemistry of thioredoxin catalysis with force. Nature450, 124–127 (2007). CASPubMedPubMed CentralGoogle Scholar
- Garcia-Manyes, S., Liang, J., Szoszkiewicz, R., Kuo, T.-L. & Fernández, J. M. Force-activated reactivity switch in a bimolecular chemical reaction. Nat. Chem.1, 236–242 (2009). CASPubMedGoogle Scholar
- Hosono, N. et al. Forced unfolding of single-chain polymeric nanoparticles. J. Am. Chem. Soc.137, 6880–6888 (2015). CASPubMedGoogle Scholar
- Levy, A., Feinstein, R. & Diesendruck, C. E. Mechanical unfolding and thermal refolding of single-chain nanoparticles using ligand–metal bonds. J. Am. Chem. Soc.141, 7256–7260 (2019). CASPubMedGoogle Scholar
- Liu, N. & Zhang, W. Feeling inter- or intramolecular interactions with the polymer chain as probe: recent progress in SMFS studies on macromolecular interactions. ChemPhysChem13, 2238–2256 (2012). CASPubMedGoogle Scholar
- Huang, Z. et al. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci.6, 2487–2495 (2018). CASPubMedPubMed CentralGoogle Scholar
- Kakkar, A., Traverso, G., Farokhzad, O. C., Weissleder, R. & Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem.1, 0063 (2017). CASGoogle Scholar
- Breslow, D. S., Edwards, E. I. & Newburg, N. R. Divinyl ether-maleic anhydride (pyran) copolymer used to demonstrate the effect of molecular weight on biological activity. Nature246, 160–162 (1973). CASPubMedGoogle Scholar
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug. Discov.2, 347–360 (2003). CASPubMedGoogle Scholar
- Zhou, D. et al. The transition from linear to highly branched poly(β-amino ester)s: branching matters for gene delivery. Sci. Adv.2, e1600102 (2016). PubMedPubMed CentralGoogle Scholar
- Wei, H., Chu, D. S. H., Zhao, J., Pahang, J. A. & Pun, S. H. Synthesis and evaluation of cyclic cationic polymers for nucleic acid delivery. ACS Macro Lett.2, 1047–1050 (2013). CASGoogle Scholar
- Cortez, M. A. et al. The synthesis of cyclic poly(ethylene imine) and exact linear analogues: an evaluation of gene delivery comparing polymer architectures. J. Am. Chem. Soc.137, 6541–6549 (2015). CASPubMedGoogle Scholar
- Newland, B. et al. GDNF gene delivery via a 2-(dimethylamino)ethyl methacrylate based cyclized knot polymer for neuronal cell applications. ACS Chem. Neurosci.4, 540–546 (2013). CASPubMedPubMed CentralGoogle Scholar
- Cook, A. B. et al. Branched poly (trimethylphosphonium ethylacrylate-co-PEGA) by RAFT: alternative to cationic polyammoniums for nucleic acid complexation. J. Interdiscip. Nanomed.3, 164–174 (2018). CASPubMedPubMed CentralGoogle Scholar
- Cho, H. Y. et al. Synthesis of biocompatible PEG-based star polymers with cationic and degradable core for siRNA delivery. Biomacromolecules12, 3478–3486 (2011). CASPubMedGoogle Scholar
- Dai, F., Sun, P., Liu, Y. & Liu, W. Redox-cleavable star cationic PDMAEMA by arm-first approach of ATRP as a nonviral vector for gene delivery. Biomaterials31, 559–569 (2010). CASPubMedGoogle Scholar
- Zhao, T. et al. Significance of branching for transfection: synthesis of highly branched degradable functional poly(dimethylaminoethyl methacrylate) by vinyl oligomer combination. Angew. Chem. Int. Ed.53, 6095–6100 (2014). CASGoogle Scholar
- Jackson, A. W. et al. Synthesis and in vivo magnetic resonance imaging evaluation of biocompatible branched copolymer nanocontrast agents. Int. J. Nanomed.10, 5895–5907 (2015). CASGoogle Scholar
- Hatton, F. L. et al. Hyperbranched polydendrons: a new nanomaterials platform with tuneable permeation through model gut epithelium. Chem. Sci.6, 326–334 (2015). CASPubMedGoogle Scholar
- Xu, Q. et al. Bacteria-resistant single chain cyclized/knotted polymer coatings. Angew. Chem. Int. Ed.58, 10616–10620 (2019). CASGoogle Scholar
- Namivandi-Zangeneh, R. et al. The effects of polymer topology and chain length on the antimicrobial activity and hemocompatibility of amphiphilic ternary copolymers. Polym. Chem.9, 1735–1744 (2018). CASGoogle Scholar
- Rolfe, B. E. et al. Multimodal polymer nanoparticles with combined 19 F magnetic resonance and optical detection for tunable, targeted, multimodal imaging in vivo. J. Am. Chem. Soc.136, 2413–2419 (2014). CASPubMedGoogle Scholar
- Tai, H. et al. Thermoresponsive and photocrosslinkable PEGMEMA-PPGMA-EGDMA copolymers from a one-step ATRP synthesis. Biomacromolecules10, 822–828 (2009). CASPubMedGoogle Scholar
- Dong, Y. et al. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv. Funct. Mater.27, 1606619 (2017). Google Scholar
- Sigen, A. et al. Hyperbranched PEG-based multi-NHS polymer and bioconjugation with BSA. Polym. Chem.8, 1283–1287 (2017). Google Scholar
- Chen, X. et al. Conformational manipulation of scale-up prepared single-chain polymeric nanogels for multiscale regulation of cells. Nat. Commun.10, 2705 (2019). PubMedPubMed CentralGoogle Scholar
- Zhao, T., Sellers, D. L., Cheng, Y., Horner, P. J. & Pun, S. H. Tunable, injectable hydrogels based on peptide-cross-linked, cyclized polymer nanoparticles for neural progenitor cell delivery. Biomacromolecules18, 2723–2731 (2017). CASPubMedGoogle Scholar
- Yoshimatsu, K. et al. Temperature-responsive “catch and release” of proteins by using multifunctional polymer-based nanoparticles. Angew. Chem. Int. Ed.51, 2405–2408 (2012). CASGoogle Scholar
- Lee, S. H. et al. Engineered synthetic polymer nanoparticles as IgG affinity ligands. J. Am. Chem. Soc.134, 15765–15772 (2012). CASPubMedPubMed CentralGoogle Scholar
- Hoshino, Y. et al. Recognition, neutralization, and clearance of target peptides in the bloodstream of living mice by molecularly imprinted polymer nanoparticles: a plastic antibody. J. Am. Chem. Soc.132, 6644–6645 (2010). CASPubMedPubMed CentralGoogle Scholar
- Pan, G., Guo, Q., Ma, Y., Yang, H. & Li, B. Thermo-responsive hydrogel layers imprinted with RGDS peptide: a system for harvesting cell sheets. Angew. Chem. Int. Ed.52, 6907–6911 (2013). CASGoogle Scholar
- Ma, Y., Pan, G., Zhang, Y., Guo, X. & Zhang, H. Narrowly dispersed hydrophilic molecularly imprinted polymer nanoparticles for efficient molecular recognition in real aqueous samples including river water, milk, and bovine serum. Angew. Chem. Int. Ed.52, 1511–1514 (2013). CASGoogle Scholar
- Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science324, 59–63 (2009). CASPubMedPubMed CentralGoogle Scholar
- Hook, A. L. et al. Combinatorial discovery of polymers resistant to bacterial attachment. Nat. Biotechnol.30, 868–875 (2012). CASPubMedPubMed CentralGoogle Scholar
- Vining, K. H. et al. Synthetic light-curable polymeric materials provide a supportive niche for dental pulp stem cells. Adv. Mater.30, 1704486 (2018). Google Scholar
- Mei, Y. et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater.9, 768–778 (2010). CASPubMedPubMed CentralGoogle Scholar
- Yan, M. et al. Single siRNA nanocapsules for enhanced RNAi delivery. J. Am. Chem. Soc.134, 13542–13545 (2012). CASPubMedPubMed CentralGoogle Scholar
- Yan, M. et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat. Nanotechnol.5, 48–53 (2010). CASPubMedGoogle Scholar
- Koide, H. et al. A polymer nanoparticle with engineered affinity for a vascular endothelial growth factor (VEGF165). Nat. Chem.9, 715–722 (2017). CASPubMedGoogle Scholar
- Tillberg, P. W. et al. Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies. Nat. Biotechnol.34, 987–992 (2016). CASPubMedPubMed CentralGoogle Scholar
- Chen, F. et al. Nanoscale imaging of RNA with expansion microscopy. Nat. Methods13, 679–684 (2016). CASPubMedPubMed CentralGoogle Scholar
- Zhao, Y. et al. Nanoscale imaging of clinical specimens using pathology-optimized expansion microscopy. Nat. Biotechnol.35, 757–764 (2017). CASPubMedPubMed CentralGoogle Scholar
- Tang, W. & Matyjaszewski, K. Kinetic modeling of normal ATRP, normal ATRP with [Cu II ]0, reverse ATRP and SR&NI ATRP. Macromol. Theory Simul.17, 359–375 (2008). CASGoogle Scholar
- Soeriyadi, A. H. et al. Synthesis and modification of thermoresponsive poly(oligo(ethylene glycol) methacrylate) via catalytic chain transfer polymerization and thiol–ene Michael addition. Polym. Chem.2, 815–822 (2011). CASGoogle Scholar
- Baudry, R. & Sherrington, D. C. Facile synthesis of branched poly(vinyl alcohol)s. Macromolecules39, 5230–5237 (2006). CASGoogle Scholar
- Bao, Y., Shen, G., Liu, X. & Li, Y. RAFT polymerization of a novel allene-derived asymmetrical divinyl monomer: a facile strategy to alkene-functionalized hyperbranched vinyl polymers with high degrees of branching. J. Polym. Sci. A Polym. Chem.51, 2959–2969 (2013). CASGoogle Scholar
- Jia, Y.-B. et al. Controlled divinyl monomer polymerization mediated by Lewis pairs: a powerful synthetic strategy for functional polymers. ACS Macro Lett.3, 896–899 (2014). CASGoogle Scholar
- Butler, G. B. & Myers, G. R. The fundamental basis for cyclopolymerization. IV. Radiation initiated solid-state polymerization of certain dimethacrylamides. J. Macromol. Sci. A. Chem.5, 135–166 (1971). Google Scholar
- Costa, A. I., Barata, P. D. & Prata, J. V. Radical cyclopolymerization of a divinylbenzyl-p-tert-butylcalix[4]arene derivative. React. Funct. Polym.66, 465–470 (2006). CASGoogle Scholar
- Edizer, S. et al. Efficient free-radical cyclopolymerization of oriented styrenic difunctional monomers. Macromolecules42, 1860–1866 (2009). CASGoogle Scholar
- Ochiai, B., Ito, S. & Endo, T. Chiral interaction between aromatic aldehydes and a polymer bearing large chiral rings obtained by cyclopolymerization of bisacrylamide. Polym. J.42, 138–141 (2010). CASGoogle Scholar
- Sharma, A. K., Cornaggia, C. & Pasini, D. Controlled RAFT cyclopolymerization of oriented styrenic difunctional monomers. Macromol. Chem. Phys.211, 2254–2259 (2010). CASGoogle Scholar
Acknowledgements
The authors acknowledge the Science Foundation Ireland (SFI) Principal Investigator (PI) Programme (13/IA/1962) (to W.W.), National Science Foundation (NSF) Division of Materials Research (DMR) (1501324) (to K.M.), National Natural Science Foundation of China (NSFC) (51873179) (to W.W.), Senior Visiting Scholarship of State Key Laboratory of Molecular Engineering of Polymers, Fudan University (19FGJ07) (to W.W.), Irish Research Council (IRC) Employment-Based Postgraduate Programme (EBPPG/2018/159) (to J.L.) and University College Dublin (to Y.G.) for financial support. The authors apologize to those whose work is relevant but could not be cited owing to space limitations.
Author information
- Yongsheng Gao Present address: School of Engineering and Applied Sciences, Harvard University, Cambridge, MA, USA
Authors and Affiliations
- Charles Institute of Dermatology, School of Medicine, University College Dublin, Dublin, Ireland Yongsheng Gao, Dezhong Zhou, Jing Lyu, Sigen A, Qian Xu & Wenxin Wang
- School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an, Shaanxi, China Dezhong Zhou
- School of Mechanical and Materials Engineering, University College Dublin, Dublin, Ireland Jing Lyu & Wenxin Wang
- School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, UK Ben Newland
- Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA, USA Krzysztof Matyjaszewski
- School of Chemistry, Bangor University, Deiniol Road, Bangor, Gwynedd, UK Hongyun Tai
- State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, China Wenxin Wang
- Yongsheng Gao












